Abstract
Introduction: The phase 3 A.R.R.O.W. study (NCT02412878) has shown a favorable benefit-risk profile of once-weekly carfilzomib (K) at 70 mg/m2 over twice-weekly K at 27mg/m2 plus dexamethasone (d) for the treatment of patients (pts) with relapsed and refractory multiple myeloma (RRMM) (Moreau. Lancet Oncol. 2018;19:953-964). Once-weekly significantly improved progression-free survival (PFS) compared with twice-weekly (median 11.2 vs 7.6 months [mos]; hazard ratio [HR]: 0.69; 95% confidence interval [CI]: 0.54, 0.88; P=0.0014). Herein, we present a subgroup analysis of once-weekly vs twice-weekly Kd by previous therapy in pts with RRMM.
Methods: 478 pts with RRMM, previously treated with 2 or 3 prior therapies including a proteasome inhibitor and an immunomodulatory agent were randomized 1:1 to receive either once-weekly (n=240) or twice-weekly (n=238) Kd. The once-weekly group received K (30-min intravenous infusion [IV]) on days (D) 1, 8, and 15 of all cycles (20 mg/m2 on D1 [cycle 1]; 70 mg/m2 thereafter). The twice-weekly group received K (10-min IV) on D1, 2, 8, 9, 15 and 16 (20 mg/m2 on D1 and 2 during cycle 1 and 27 mg/m2 thereafter). All pts received d at 40 mg/m2 on D1, 8, 15 (all cycles), and 22 (cycle 1-9 only). Treatment was given in 28-day cycles until disease progression or unacceptably toxicity. The primary endpoint was progression-free survival (PFS). The study secondary endpoints were overall response rate (ORR), survival, safety, and pharmacokinetics.
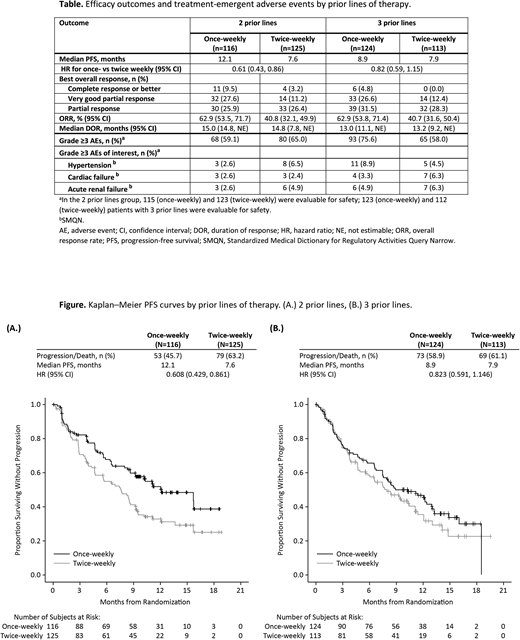
Results: Baseline characteristics were generally balanced. PFS, ORR, and safety outcomes by prior lines of therapy are shown in the Table; PFS curves by prior lines are shown in the Figure. With a median follow-up time of 12.6 mos in the once-weekly group and 12.0 mos in the twice-week group, PFS for pts with 2 prior lines (N=241) was 12.1 mos vs 7.6 mos (HR: 0.61; 95% CI: 0.43, 0.86). Median PFS for pts with 3 prior lines (N=237) was 8.9 mos for once-weekly vs 7.9 mos for twice-weekly (HR: 0.82; 95% CI: 0.59, 1.15). ORRs for once- vs twice-weekly were 62.9% vs 40.8% (OR: 2.46; 95% CI: 1.47, 4.14) in pts with 2 prior lines and 62.9% vs 40.7% (OR: 2.47; 95% CI: 1.46, 4.17) in pts with 3 prior lines. In the 2 prior lines group, 9.5% vs 3.2% of pts had a complete response or better with once- vs twice-weekly Kd respectively. Complete responses or better in pts with 3 prior lines were obtained in 4.8% of pts receiving once-weekly and no pts with twice-weekly Kd. In A.R.R.O.W., 401 (83.9%) pts had prior lenalidomide (LEN) exposure. Median PFS for once- vs twice-weekly was 11.1 mos vs 7.4 mos (HR: 0.72; 95% CI: 0.56, 0.94) for LEN exposed pts and NE (not estimable) vs 9.4 mos (HR: 0.63; 95% CI: 0.33, 1.21) for pts without prior LEN exposure. ORRs for once- vs twice-weekly were 62.3% vs 39.2% (OR: 2.57; 95% CI: 1.72, 3.84) in pts with prior LEN exposure, and 66.7% vs 47.7% (OR: 2.19; 95% CI: (0.86, 5.58) in pts without prior LEN exposure.
Grade ≥3 treatment-emergent adverse events (AEs) occurred in 59.1% vs 65.0% (once- vs twice-weekly) of pts with 2 prior lines of therapy, and 75.6% vs 58.0% (once- vs. twice-weekly) of pts with 3 prior lines of therapy. The incidence of Grade ≥3 AEs of interest by prior lines can be found in the Table. Grade ≥3 AEs occurred in 69.3% vs 60.2% (once- vs twice-weekly) of pts with prior LEN, and 57.6% vs 68.2% (once- vs. twice-weekly) of pts without prior LEN.
Conclusions: Once-weekly Kd resulted in a favorable benefit-risk profile regardless of the number of prior lines. Although the median PFS was prolonged in both groups, pts with 2 prior lines achieved a greater benefit, underlining the need for using once-weekly Kd early to optimize outcomes for pts with RRMM. Safety profiles were consistent with previous findings and reports. Importantly, low incidences of heart failure were reported across all subgroups and no additional toxicities were found. As described for other K-based regimens, efficacy of K can be further enhanced by being used in earlier lines of therapy for pts with RRMM. This subgroup analysis further confirmed the positive results from A.R.R.O.W. of using once-weekly Kd at 70 mg/m2 regardless of prior therapies and should be considered an additional option to treat pts with RRMM.
Moreau:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Stewart:Amgen Inc., Celgene, Roche, Seattle Genetics: Research Funding; Amgen Inc., BMS, Celgene, Takeda, Roche, Seattle Genetics, Janssen, Ono: Consultancy. Lazzaro:Celgene: Consultancy, Research Funding; Amgen: Research Funding. Dimopoulos:Janssen: Honoraria; Amgen: Honoraria; Celgene: Honoraria; Takeda: Honoraria; Bristol-Myers Squibb: Honoraria. Cavo:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ailawadhi:Amgen: Consultancy; Pharmacyclics: Research Funding; Takeda: Consultancy; Janssen: Consultancy; Celgene: Consultancy. Iskander:Amgen: Employment, Equity Ownership. Huang:Amgen: Employment, Equity Ownership. Zahlten-Kumeli:Amgen: Employment, Equity Ownership. Mateos:GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal